In researching these materials we discovered a wide variety of conflicting and, at times, just plain wrong information about the age-old process of alcohol distillation. In this first installment on our in-depth and continually expanding informational resource on Probrewer.com, we have and will continue to strive to present the brewing industry with the most accurate and up-to-date information available.
Sample Spirit Recipes
The World of Benefits
Brewers: We don’t have to tell you that beer, with its 85% points of profit, is a good business, but did you know: with $150 worth of malt you can get a volume of two-hundred 0.7 liter bottles of strong whiskey?
At $8 per bottle, your profit is approximately $1450, or 90% profit. Some producers are selling as high as $30 to $70 per bottle. Similar opportunities exist with beer-brandy, as well (distilled beer, also called beer-schnapps).
Growing or fruit-packing companies: Prunes, apricots, cherries, pears or apples-many times you don’t know what to do with your left over fruit which is not good for direct consumption or other types of processing because they are undersized or too ripe. Two thousand pounds of prunes valued at $0.10 or less per pound may yield 250 bottles of fruit brandy that sells in Europe (and now in some places in the U.S.) for $20 to $70 per bottle. At $6.00 profit per bottle, you can make $1500 with a $200 investment in raw materials.
Wineries: Vineyards often end up with wines they feel are not of sufficient quality for supermarket shelves. These can be converted into a Cognac style brandy or even into your own port wine to add to your product line. Or a trendy grappa from grape residuals not useful for wine.
The opportunities are endless. Thinking back to the success of the beer-revolution of the past 20 years, distilling could very well be the next boom industry.
What is distillation?
Distill- Dis*till”\, v. t.
1. To let fall or send down in drops.
2. To obtain by distillation; to extract by distillation, as spirits, essential oil, etc.; to rectify; as, to distill brandy from wine; to distill alcoholic spirits from grain; to distill essential oils from flowers, etc.; to distill fresh water from sea water.
3. To subject to distillation; as, to distill molasses in making rum; to distill barley, rye, corn, etc.
Source: Webster’s Revised Unabridged Dictionary, © 1996, 1998 MICRA, Inc.
The Distillation Process
In the most basic sense, distillation is a process used to separate a composite mixture into base elements. It involves a change of state, usually from liquid to gas, and then subsequent condensation to return the now separated elements to liquid state.
The basic distillation apparatus (still) has three parts:
(1) A flask with an outlet tube,
(2) A condenser, and
(3) A vessel.
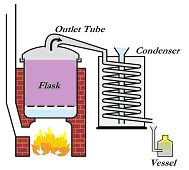
Distillation Simplified
Distillation has been and is used to separate alcohol from various fruits, grains, wines and beers. Once the raw material (fruits, grains, etc) has fermented it is heated. Since different base elements in the material, such as water, alcohol, and fusel oils have varying boiling points, the elements separate. The distilled material is then collected in a vessel – with care that the heads and tails (undesirable elements) are excluded from the drinkable alcohol.
Types of Stills Used in the Spirit Industry
Alembic Pot still – The oldest and most recognized still design. The flask or kettle is typically copper and resembles a huge onion shape, which liberates the alcohol from the mixture. The vapors rise and pass through a narrow pipe and then through a serpentine coil, a cold-water bath condenses the vapors in the coils, converting them back to liquid form.
Reflux or Column still – A ‘technological’ advance over the pot still. It is more efficient, requiring only a single distillation done in one continuous operation. This type of still allows for exact separation techniques. Also, changing the reflux rate provides great flexibility to create the style and quality of the type of spirit produced.
The reflux still is not only more efficient, but also is equipped to reduce potential cyanides and ethylcarbamate that are harmful if too much of them are present.
Other Considerations
Water consumption per batch: For every 600 liters of batch, estimate about 1200 liters of water needed. The water is required for cooling and should not exceed an inlet temperature of less than 20 °C. The water outlet temperature is ~ 70 °C. If the costs for water are high, there are technical solutions to circulate chilled water. The temperature of the output distillate should be about 20°C.
Steam consumption: about 100 kg/hr is the standard value for a capacity of 600 liter, assuming 1-hour time to bring the mash to boil. To finish the process, you need about an additional 1.5 hours consuming approximately 70 kg steam per hour.
For the calculation of the energy consumption the following basic numbers are valid: Net 170,000 BTU is required per hour. For gas fired steam boilers this means a 220,000 BTU on input. Based on the BTU value of your natural gas or propane, you can calculate to cost of fuel.
Origins and History of Process
Distillation, the process of separating a liquid into different parts by evaporation and condensation, is an age-old process that may have began as early as 2000 BC. Some say that the first use of distillation occurred in China, Egypt, or Mesopotamia for medicinal purposes as well as to create balms, essences, and perfumes. Over time, the secrets of the distillation process traversed thousands of miles, moving over Europe, crossing England, Scotland and Ireland, and settling in the Americas. [Read more]
Terminology
Familiarize yourself with the distillery business vocabulary, from Aqua Vitae to Saccharification.
Legal considerations
For up to date information regarding State and Federal guidelines for your area refer to the following websites.
– TTB (formerly ATF) – Distilled Spirits Regulations
– State Laws
– EPA
– More EPA
